Select All the Intermolecular Forces Associated With 1-pentanol.
What types of intermolecular forces are present in H2 and CCl. Intermolecular Forces AIdentify the intermolecular forces present in the following substances and B select the substance with the highest boiling point.
Solved Select All The Intermolecular Forces Associated With Chegg Com
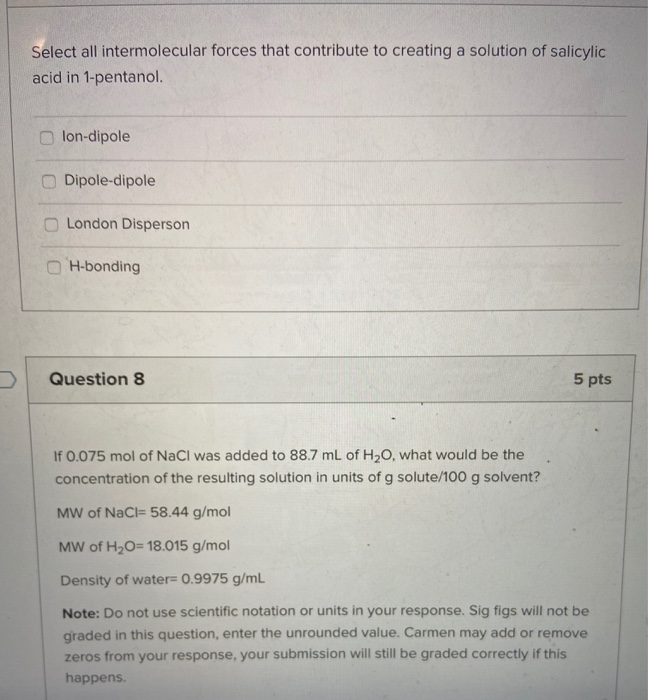
London Disperson Dipole-dipole lon-dipole H-bonding Select all intermolecular forces that contribute to creating a solution of salicylic acid in 1-pentanol.

. Select all the intermolecular forces associated with NaCl salt. Compounds with strong intermolecular forces have high boiling points. Intermolecular forces from weakest to strongest.
Explain the differences in boiling point of a 1-pentene bp 30C 1-bromopentane bp 129C and 1-pentanol bp 137C. Is 1 Pentanol polar or nonpolar. Follow the links above to find out more about the data in these sites and their terms of usage.
1-3 The dielectric relaxation studies take a vital role to elucidate the nature of interactions in a liquid system with polar and non polar molecules. Identify the types of intermolecular forces present in each of the following substances and select the substance in each pair that has the higher boiling point. What is the typical mp range of a pure compound.
Asked Sep 17 2016 in Chemistry by Garixx. Forces dipole-dipole forces and hydrogen. For example water with its strong intermolecular hydrogen bonding has one of the highest surface tension values of any liquid whereas low-boiling-point organic molecules which have relatively weak intermolecular forces have much lower surface tensions.
Lon-dipole Dipole-dipole London Disperson H-bonding Question 8 5 pts If 0075 mol of NaCl was added to 887 mL of H20 what would be the. Ethanol and acetic acid have hydrogen bonding. O A good recrystallization solvent has a structure that is.
C Compounds are made up of two or more different types of atoms D Compounds can be isolated in pure form. Van der Waals forces dipole - induced dipole dipole - dipole dipole - ion ion -. Evaporation requires the breaking of all intermolecular forces.
What forces are present in pentane. CH 3CH 3 CH 3OH and CH 3CH 2OH Answers. Does 1-propanol have dipole-dipole forces.
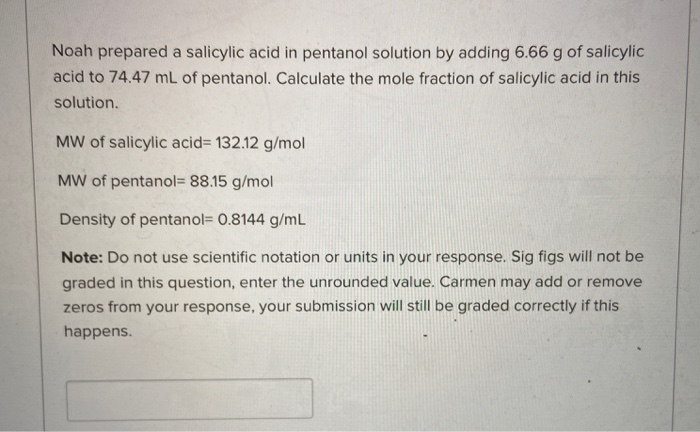
A CH 3CH 3 has only dispersion forces whereas the other two substances have both dispersion forces and hydrogen bonds. MW of salicylic acid 13812 gmol MW of pentanol 8815 gmol Density of. D Only hydrogen bonding forces are present.
Lon-dipole Dipole-dipole H-bonding London Disperson If 0066 mol of salicylic acid was added to 6578 mL of pentanol what would be the concentration of the resulting solution in units of g solute100 g solvent. Your institution may already be a subscriber. B CH 3CH 2OH.
Intermolecular forces 1-pentanol London dispersion forces and H-bonding pentane London dispersion forces 1-Pentanol should have larger intermolecular forces due to H-bonding meaning the molecules are more attracted to each other than in pentane. Pentanol you found that crotonic acid was most. Evaporation requires the breaking of all intermolecular forces.
Methanol Pentanol We usually consider hydrocarbon chains to be non-polar when discussing solubility and so overall Methanol and Pentanol are hybrids they have a polar group on one end and a non-polar group on the other. O2 dispersion forces dipole-dipole forces dispersion forces and dipole-dipole. A Dispersion hydrogen bonding and dipole-dipole forces are present.
1-Pentanol should have larger intermolecular forces due to H- bonding meaning the molecules are more attracted to each other than in pentane. Select all the intermolecular forces associated with 1-pentanol. Intermolecular interaction among the liquid mixtures takes place a vital role in chemical industries and reseaech field.
E A compound can be separated into different elements with their own unique properties. C Only dispersion and dipole-dipole forces are present. 45 Methyl formate is used in various chemical and pharmaceutical industries.
In liquid pentanol CH3CH2CH2CH2 which intermolecular forces are present. Induced dipole - induced dipole aka. What are the features of a good recrystallization solvent.
Intermolecular forces are required to make molecules stick together and they are the reason why compounds with differing. 1 pentanol solubility in water. A Compounds have characteristic physical properties.
Hence the following intermolecular forces are present in 1-pentanol. This is because the strength of the intermolecular forces increases holding the. B Compounds have different chemical properties than the elements that compose them.
Octane and pentane have only London dispersion forces. O Typically 05 - 15 centigrade. Thus the water molecule exhibits two.
117-118 C Alfa Aesar. Ion-dipole forces are the forces responsible for the solvation of ionic compounds in aqueous solutions and are the strongest of the intermolecular foces. O High molecular symmetry and larger molecular surface area are associated with greater intermolecular forces and higher melting points.
What intermolecular forces does 1-Pentanol have. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. It would take more energy to.
B Only dipole-dipole and ion-dipole forces are present.

Solved Select All The Intermolecular Forces Associated With Chegg Com
Solved Select All The Intermolecular Forces Associated With Chegg Com
Solved Select All The Intermolecular Forces Associated With Chegg Com
No comments for "Select All the Intermolecular Forces Associated With 1-pentanol."
Post a Comment